Naming Aromatics
Key Questions
-
Let's just look at two simple examples:
- o-bromotoluene
Where does it come from? The IUPAC name for this is 1-bromo-2-methylbenzene because you go clockwise and start at 1. Why is it called o-bromotoluene as well? Toluene is just a common name for 1-methylbenzene. "o" means ortho, which means "next to". Bromine is next to the methyl group, and bromo is the substituent name for bromine.
If bromine were on carbon 3, it would be called m-bromotoluene, with "m" for meta ("after"). If Bromine were on carbon 4, it would be called p-bromotoluene, with "p" for para ("opposite [side] to").
You can infer further. If you have three substituents, just find the first one alphabetically, then the second, and so on. Minimize the carbon numbering magnitudes (i.e. 1, 2, 4 is better than 2, 3, 5).
This would be 1-bromo-2-chloro-3-fluorobenzene.
And lastly, some common aromatic compounds that would be nice to know because you might see them often:
- Toluene (1-methylbenzene, or phenylmethane)
- Styrene (1-ethenylbenzene, or phenylethylene)
- Anisole (1-methoxybenzene, or phenyl methyl ether)
- Aniline (1-aminobenzene, or phenylamine)
- Phenol (1-hydroxybenzene, or phenyl hydroxide)
- o-bromotoluene
-
An aromatic hydrocarbon is a hydrocarbon that contains
#4n+2# electrons in a planar conjugated π system (#n = 0, 1, 2, …# ).The most common aromatic compounds contain benzene rings (
#n = 1# ).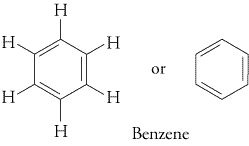
Examples are toluene and trinitrotoluene (TNT).
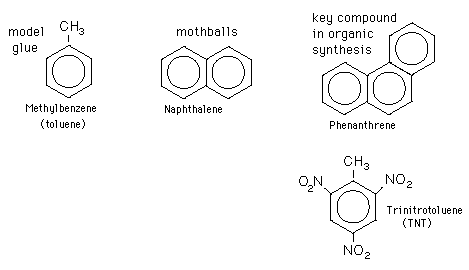
But any planar cyclic hydrocarbon that contains 2, 6, 10, 14 … π electrons is aromatic.
Examples are naphthalene (10 π electrons) and phenanthrene (14 π electrons).
The ring doesn't have to contain a 6-membered ring, as long as it is planar contains
#4n+2# π electronsThus, [18]annulene is aromatic. It is planar and possesses
#4n+2# π electrons (with#n = 4# ).