What are aromatic hydrocarbons?
1 Answer
May 7, 2015
An aromatic hydrocarbon is a hydrocarbon that contains
The most common aromatic compounds contain benzene rings (
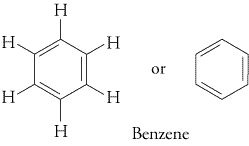
Examples are toluene and trinitrotoluene (TNT).
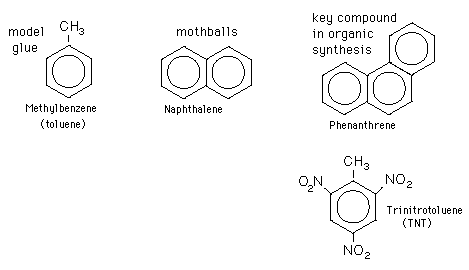
But any planar cyclic hydrocarbon that contains 2, 6, 10, 14 … π electrons is aromatic.
Examples are naphthalene (10 π electrons) and phenanthrene (14 π electrons).
The ring doesn't have to contain a 6-membered ring, as long as it is planar contains
Thus, [18]annulene is aromatic. It is planar and possesses


