Hydrogenation
Key Questions
-
Yes they are.
Many oils are unsaturated, that means they have double bonds in the chain structure of their fatty acids. Double bonds tend to make the chains less flexible, so they can't fit together nicely and attract each other less well by London forces.
In short: unsaturated fats tend to have a lower melting point, and are usually called oils (the difference between oil and fat is not really chemical, it's just the state they're in at room temperature).
By hydrogenation, we add a hydrogen atom at both sides of a double bond:
#-CH=CH- +H_2->-CH_2-CH_2-# So it becomes a single bond, and heightens the melting point. This is not only done to vegetable oil, but to any oil that needs to "harden" (like fish oil).
Below is an example of a fat (or oil) with the usual three fatty acid chains: one saturated (blue) one unsaturated (green) and one poly-unsaturated (red). The black part in the middle is the glyceride-group that holds the lot together. -
Answer:
Not necessarily.
Explanation:
If you add hydrogen molecules to a compound, nothing will happen most of the time.
If you add hydrogen ions to a compound, generally it will reduce the compound and create a compound that contains a hydrogen bonded to it.
If you add a hydride anion to a compound, generally it will oxidise the compound and create a compound that contains a hydrogen bonded to it.Having only one valence electron, hydrogen is very flexible and experiences virtually no shielding effect from its nucleus. Therefore, hydrogen is often caught up in dative bonds as well as other types (like ionic or covalent bonds).
Therefore, the addition of hydrogen to a compound may not always result in a reduction reaction. You may also want to read up on hydrogenation. Hope this helps!
-
Hydrogenation requires a catalyst to make the reaction go at a reasonable rate.
The reaction will go without a catalyst , but it needs extremely high temperatures.
Consider the reaction: CH₂=CH₂ + H-H → CH₃-CH₃.
We must break the π bond and the H-H σ bond to form the two new C-H bonds.
The π bond is relatively weak, but the H-H bond is quite strong.
A metal catalyst provides an alternate pathway with a lower activation energy. This allows the reaction to take place at lower temperatures.
We don't know the details of catalytic hydrogenation with Ni (or Pt or Pd).
We believe that when hydrogen and the alkene are adsorbed onto the catalyst, they bind to the surface of its crystal lattice.
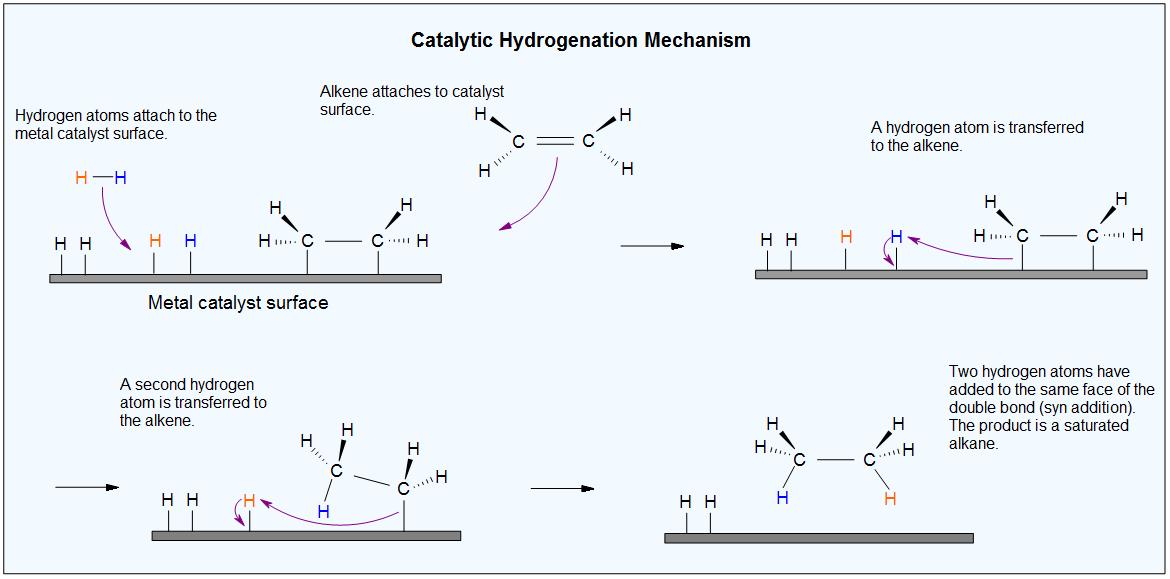
The H-H bond may break and form Ni-H bonds. The alkene may also break its π bond and form Ni-C bonds.
An H atom then adds to one end of the alkene. Then the other end of the alkene attaches to a second H atom.
The alkane has only a small attraction to the nickel, so it is desorbed from the surface.
This creates a vacant space for the adsorption of new alkene and hydrogen molecules, and the process continues.