Why does hydrogenation require a catalyst?
1 Answer
Hydrogenation requires a catalyst to make the reaction go at a reasonable rate.
The reaction will go without a catalyst , but it needs extremely high temperatures.
Consider the reaction: CH₂=CH₂ + H-H → CH₃-CH₃.
We must break the π bond and the H-H σ bond to form the two new C-H bonds.
The π bond is relatively weak, but the H-H bond is quite strong.
A metal catalyst provides an alternate pathway with a lower activation energy. This allows the reaction to take place at lower temperatures.
We don't know the details of catalytic hydrogenation with Ni (or Pt or Pd).
We believe that when hydrogen and the alkene are adsorbed onto the catalyst, they bind to the surface of its crystal lattice.
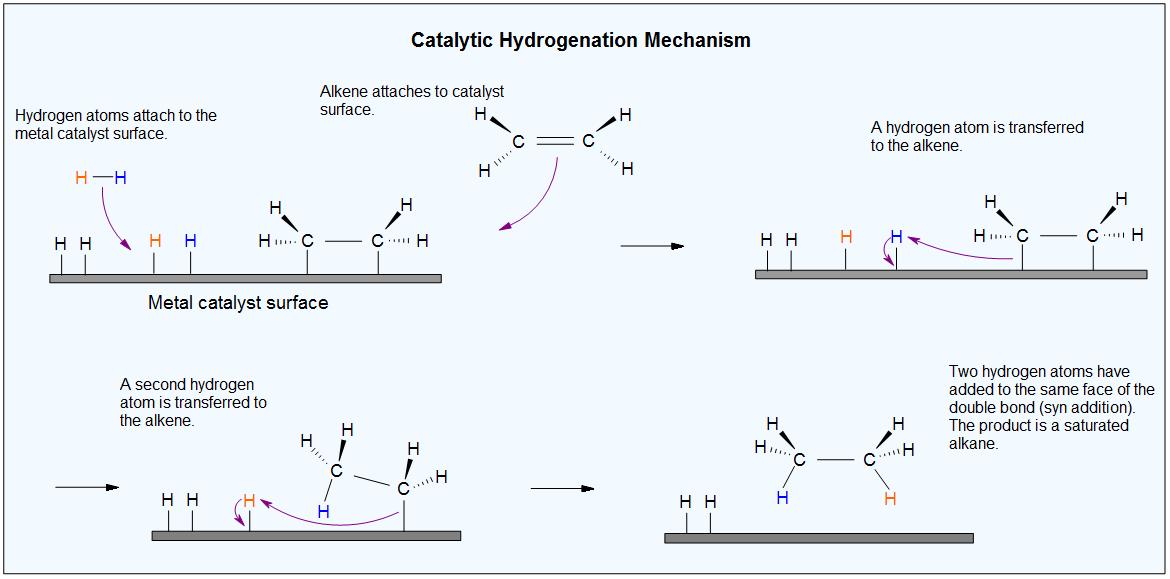
The H-H bond may break and form Ni-H bonds. The alkene may also break its π bond and form Ni-C bonds.
An H atom then adds to one end of the alkene. Then the other end of the alkene attaches to a second H atom.
The alkane has only a small attraction to the nickel, so it is desorbed from the surface.
This creates a vacant space for the adsorption of new alkene and hydrogen molecules, and the process continues.

