Acid Catalyzed Hydro-alkoxy Addition
Key Questions
-
The acid-catalyzed alkoxylation is an analogous reaction to the acid-catalyzed hydration (Markovnikov addition of water via acid catalysis), and can go as follows for a substituted alkene and a generic alcohol:
where the racemic mixture of the major or minor products can be written as a line bond instead of both the hash and wedge bonds.
(Had it not been racemic but uneven, a squiggly bond would have been the way to write it.)
The mechanism would go as follows:
with Markovnikov addition giving the major product.
- Protonation of the alkene to create reaction conditions in which an alcohol can be a good nucleophile.
- Nucleophilic backside-attack of the carbocation intermediate.
- Removal of the attached alkoxide's proton (
#"pKa" ~~ -3.6# ) to regenerate the catalyst and form the product(s).
-
An acid catalyzed hydro-alkoxy addition is the addition of an alcohol to a C=C double bond to form an ether.
An example is the addition of methanol to 2-methylpropene to form t-butyl methyl ether.

This is an electrophilic addition reaction.
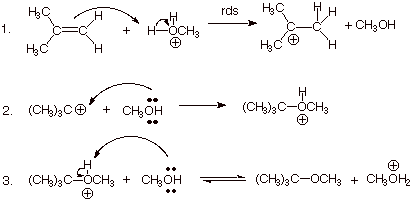
In Step 1, a hydronium or oxonium ion is attacked by the π bond.
In Step 2, the alcohol attacks the carbocation and forms an oxonium ion.
In Step 3, the alcohol deprotonates the oxonium ion to form the ether.
Here’s a video on the addition of alcohols to alkenes.