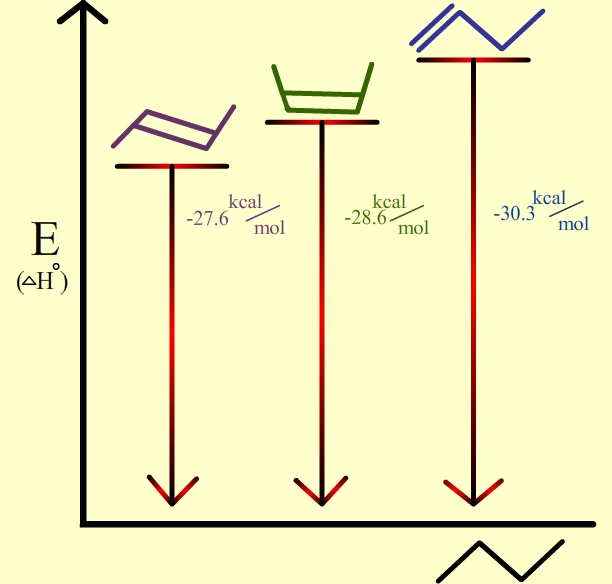
Why do the most stable alkenes have the smallest heat of hydrogenation?
1 Answer
Jan 15, 2015
The most stable alkenes have the smallest heat of hydrogenation because they are already at a low energy level.
When you hydrogenate an alkene, you get an alkane. The alkane is more stable than the alkene, so energy is released. This energy is called the heat of hydrogenation.
The diagram below shows three alkenes. All of them give the same alkane on hydrogenation.
The most stable of these alkenes is the one on the left.
It is at the lowest energy level of the three. So it releases the least energy when it is hydrogenated.