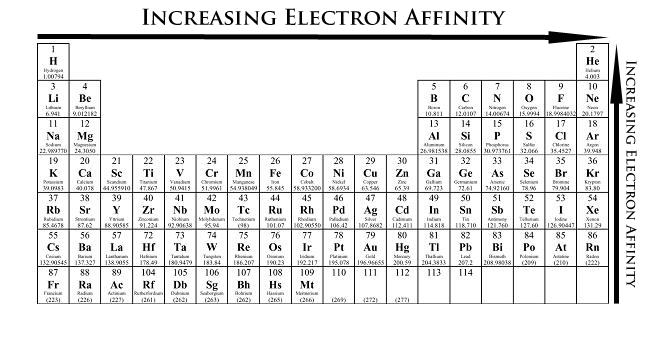
Periodic Trends in Electron Affinity
Key Questions
-
Electron affinity EA measures the energy released when an electron adds to a gaseous atom. For example,
Cl(g) + e⁻ → Cl⁻(g); EA = -349 kJ/mol
The negative sign shows that the process releases energy.
Adding an electron to a metal requires energy. Metals are much more likely to give up their electrons. Thus, metals have positive electron affinities. For example,
Na(g) + e⁻ → Na⁻(g); EA = 53 kJ/mol
In the Periodic Table, electron affinity increases (becomes more negative) from left to right in a period. Electron affinity decreases from top to bottom in a Group. But there are some exceptions.