The Periodic Table
Key Questions
-
The elements in the Periodic Table are arranged according to increasing atomic number.
As you go horizontally from left to right across a Period in the Periodic Table, you are adding one more proton to the nucleus (increasing the atomic number by one).
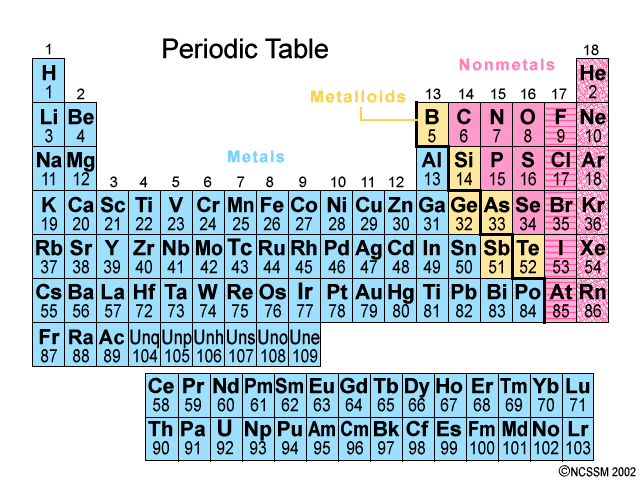
Elements with similar properties are arranged one above the other in vertical Groups numbered from 1 to 18.
Metals (blue) are on the left; nonmetals (pink) are on the right; metalloids (yellow) lie along the zigzag line that divides the metals and nonmetals. The noble gases are on the far right.
-
Answer:
The periodic table is a useful tool because it arranges all the elements in an organized and informative manner.
Explanation:
The periodic table arranges the elements into families and periods (vertical and horizontal rows). The elements in each family have similar properties. As you go across a row, the properties vary gradually from one element to the next. The table tells you what elements may have similar chemical and physical properties.
The periodic table describes the atomic structure of all known elements. For instance, by looking at the periodic table, you can find out the atomic mass and the number of electrons the element has. Each element has its own separate set of such data. No two elements are the same.
This is perhaps the most useful feature of the Periodic Table. It is an excellent reference tool. In one place, you can find many properties of an element.