Question #a6d8b
1 Answer
Electron affinity increases as you go to the right and up a periodic table. The noble gases have 0 electron affinity as they are unreactive. Hence Fluorine has the highest electron affinity.
Explanation:
Electron affinity can pretty much be defined as how much an atom wants electrons to become an ion. Elements which have negative ions will want to get electrons, whereas elements with positive ions will want to lose electrons. This means that elements with negative ions will have a higher electron affinity than elements with positive ions.
As non-metals have negative ions, whereas metal ions have positive ions, non-metals will have a higher electron affinity.
Hence, as you go towards the non-metals (top right of the periodic table), electron affinity will increase as shown in the periodic table below.
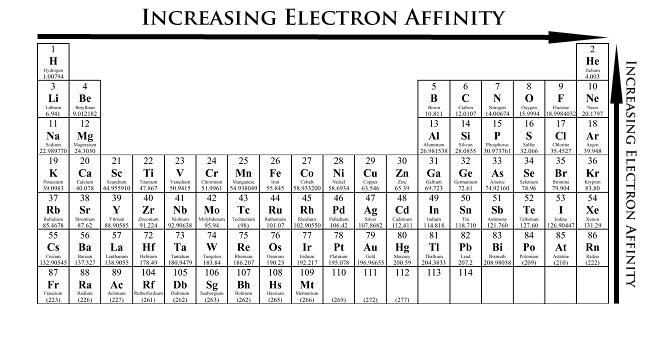
Although the noble gases are unreactive as they have full outer electron shells, hence they will not want any more electrons. This means that they have electron affinities of 0. Because of this, Fluorine will have the highest electron affinity, as it is the highest and most to the right on the periodic table (disregarding the noble gases)

