How would you order the following elements with respect to electron affinity: #Cl^-, Ar, K^+, Ti, Ti^(+2)#?
1 Answer
Explanation:
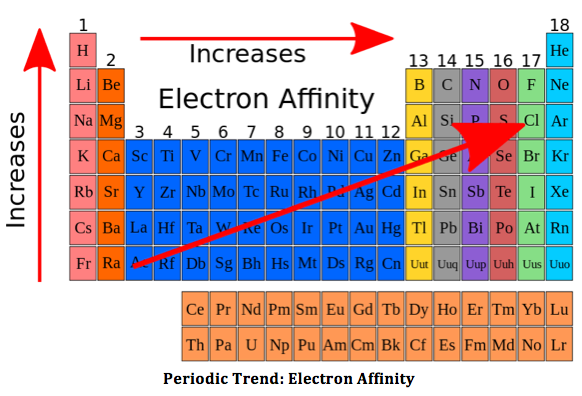
4 things affecting electron affinity
-
Shielding electrons
![Study.com]
( )
) -
Effective nuclear charge
-
distance from the nucleus
-
Noble gases will have no electron affinity as they are non-reactive
Argon will have the lowest electron affinity as it is a noble gas
Compare Cl-, K+, Ti and
Cl- has already 1 extra electron and its negative so its electron affinity will be lower than K+, Ti,
Ti can take an electron and to become negative and can release an electron to become positive so its chances are neutral. So electron affinity of Cl < Ti < K+ and
Now compare Ti+2 and K+
K+ has lost one electron there are chances that it will grab another one but Ti+2 has more chances of grabbing an electron as it has already lost 2 electrons.
So the electron affinity of these ions and elements

