Introduction to Chirality and Chiral Centers
Key Questions
-
A chiral centre is an atom that has four different groups bonded to it in such a manner that it has a nonsuperimposable mirror image.
The term "chiral centre" has been replaced by the term chirality centre.
In the molecule below, the carbon atom is a chirality centre.
It has four different groups attached, and the two structures are nonsuperimposable mirror images of each other.
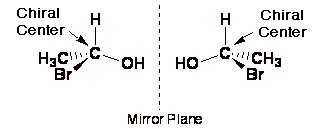
Molecules with a single chirality centre are chiral.
Molecules with more than one chirality centre are usually chiral. The exceptions are meso compounds.
For example, tartaric acid has two chirality centres, so you would expect it to have
#2^2 = 4# stereoisomers.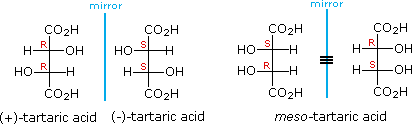
But there are only three isomers.
The (
#S,R# ) and (#R,S# ) isomers are a single meso compound because they are superimposable on each other. They are achiral because they have an internal plane of symmetry.The most common chirality centres in organic molecules are sp³ hybridized carbon atoms, because they can form four bonds.
Other types of chirality centre are
- quaternary N atoms

- tetravalent P atoms

Many nerve gases contain chiral P atoms.
- sulfoxides

In sulfoxides, the fourth group is a lone pair. Unlike in amines, the energy required to invert this stereocentre is high, so sulfoxides are optically stable.
-
Answer:
The Cahn-Ingold-Prelog sequence rules assign priorities to the groups attached to each chiral centre.
Explanation:
- The atom with higher atomic number receives higher priority.
- If there is a tie, we go one bond further out until we come to the earliest difference.
- If there is still a tie, we keep going further out until we find a difference.
- Double and triple bonds are treated as if they were separate single bonds to the same atom.
Here's an example of how to assign relative priorities.
-
Answer:
Chirality means a molecule that is mirrored won't be superimposable.
Explanation:
A chiral molecule can usually be found if there is no plane of symmetry, an example in every day life of this is your hands. (They are mirror images but one can't be put onto the other such that they would appear the same).
Picture of chiral hands:
Image from: http://spicyip.com/2015/09/patent-office-rejects-tofacitinib-patent-application-an-analysis-part-i.htmlTo apply this to molecules one must first find a specific atom whether it be a Carbon or other, where there are four different substituents bonded to the atom.
Four different substituents bonded to a chirality center:
Image from: http://glossary.periodni.com/glossary.php?en=chiral+moleculeSometimes it appears that the atom bonded directly to the atom of interest, as a chiral center, is the same as another one bonded to the atom of interest, so its necessary to look a bit further to see if substituent is indeed different from the other.
Example of this:
Image from: Created using ChemSketch 2015One can see that the
#3# indicated on the picture is a chirality center, however in order to see it you need to look at the ethyl and propyl substituents as a whole because the atoms bonded directly to the chiral Carbon are both#CH_2# .Here's a video to help your understanding:
Video from: https://www.khanacademy.org/science/organic-chemistry/stereochemistry-topic/chirality-r-s-system/v/chirality-center-jayHope I helped :)
