Intermolecular Bonds
Key Questions
-
Intermolecular forces (from Latin inter, meaning between or among) are the forces of attraction or repulsion that act between neighboring atoms, molecules, or ions.
-
Intermolecular bonds are caused by the attractive forces between the negative end of one molecule and the positive end of another.
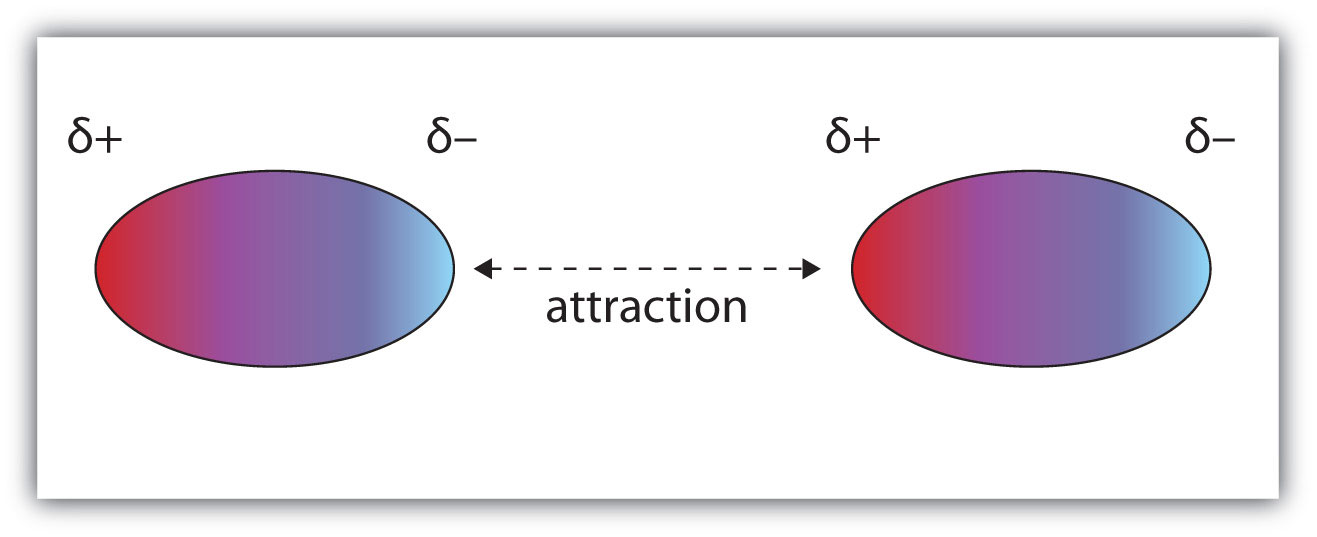
DIPOLE-DIPOLE BONDS
A polar molecule has a positive end and a negative end. When two polar molecules are near each other, they arrange themselves so that the negative and positive ends line up and attract the two molecules together
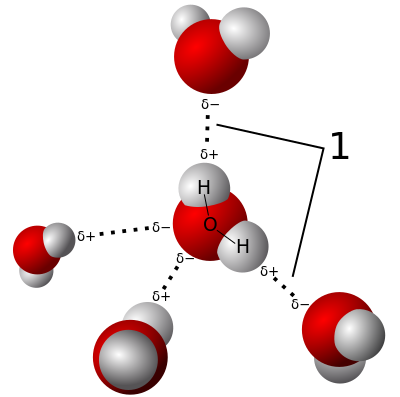
A hydrogen bond is the dipole-dipole attraction between the positive ends (the H atoms) of the O-H, N-H, and F-H bonds in one molecule and the negative ends (the N, O, or F atoms) in a neighbouring molecule.
In liquid water, for example, every water molecule can be H-bonded to four other water molecules.

There are other, weaker, attractive forces, but dipole-dipole attractions are the strongest attractions among covalent molecules.
-
Answer:
They are dipole-dipole forces, hydrogen bonds, and London dispersion forces.
Explanation:
DIPOLE-DIPOLE FORCES
Two nearby polar molecules arrange themselves so that the negative and positive ends line up. An attractive force holds the two molecules together

HYDROGEN BONDS
The H atom in an O-H, N-H, or F-H bond has a partial positive charge. The N, O, or F atoms in a neighbouring molecule have a partial positive charge.
The dipole-dipole attractions between these charges are hydrogen bonds. Water molecules have strong H-bonds.
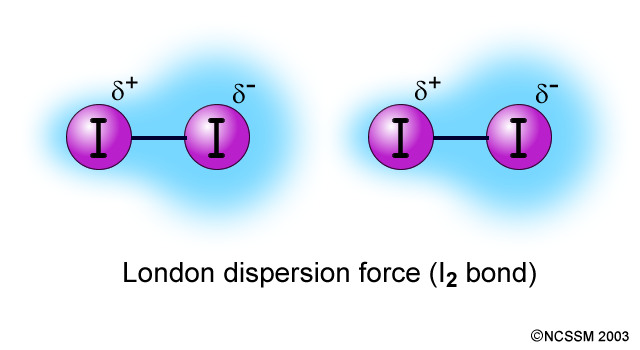
LONDON DISPERSION FORCES
At any given instant, there may be a greater electron density on one end of a nonpolar molecule than on the other. This instantaneous dipole can induce a dipole in a neighbouring molecule. This causes a weak attractive force called a London Dispersion Force.