Why is #CDCl_3# a triplet in #C^13 NMR#?
1 Answer
The deuterium in the CDCl₃ splits the
Whenever you run a
For example, here's a spectrum of t-butyl chloride.
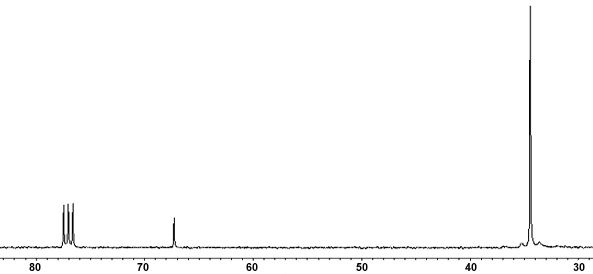
You see the characteristic CDCl₃ triplet at 77.5 ppm.
The reason is that
A nucleus with
These orientations have the same energy in the absence of a magnetic field.
In the presence of a magnetic field, these energy levels split. Each level is labelled with a magnetic quantum number
So a
Each orientation has the same probability, so the signal is a 1:1:1 triplet.


