What is the priority order of factors like resonance, hybridization, solvation, etc. in determining the relative acidity or basicity of compounds in a list?
1 Answer
WARNING! Long answer! Here are my thoughts.
Explanation:
Anything that decreases the
I would identify the factors in the order 1.
Strong acids
There are six common strong acids. In order of increasing acidity, they are:
Any strong acid is stronger than most organic acids.
Some factors to consider are:
1. Resonance
I put resonance stabilization of the anion first in the list.
The more resonance contributors a conjugate base has, the stronger the acid will be.
That partly explains why sulfuric acid is stronger than nitric acid.
It also explains why acetic acid (
ethanol (
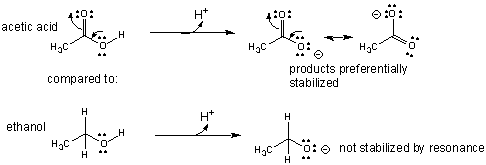
2. Atom
(a) Acidity of H-X increases from left to right on the Periodic Table.
As the electronegativity of
The exception is
(b) Acidity increases from top to bottom of the Periodic Table.
As you go down a group, the bond length becomes longer and weaker, and the charge density on the ion decreases.
3.
Electronegative atoms draw negative charge toward themselves.
This weakens the H-X bond and stabilizes the conjugate base.
The effect decreases with distance from the acidic
(From SlidePlayer)
Alkyl groups are electron-donating groups.
They push electron density onto the
The more alkyl groups, the weaker the acid.
4. Orbitals
The more
As we go from
Alkynes are remarkably acidic compared to alkanes.

Here's a summary of the discussion.

