Are aromatic amines less basic?
1 Answer
Aromatic amines are less basic than aliphatic amines.
There are three factors to consider:
- Hybridization
- Resonance
- Stability of the ions being formed
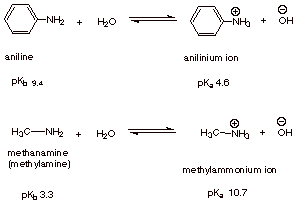
Let's compare methylamine and aniline.
Hybridization
In methylamine the C atom is
Since
They pull electrons away from the N atom.
There is less electron density on the N atom, so aniline is predicted to be a weaker base than methylamine.
Resonance
There is no resonance in methylamine, but the methyl group is electron-donating.
This increases the electron density on the N atom and enhances its basicity.
In aniline, the lone pair electrons on N are resonance-delocalized into the ring.

In addition to the two Kekulé forms, there are three contributors with electron density in the ring.
Most of the electron density is still on N, but it has been decreased somewhat, so aniline is a weaker base.
Stability of the ions
In the equilibrium
The position of equilibrium depends on the stabilities of the product ions compared with the stabilities of reactants.
The anilinium ion has only the two Kekulé resonance contributors, so it is less stable than aniline.
The position of equilibrium moves to the left, so aniline is a weaker base.