How many hybrid orbitals are there in HNO3?
1 Answer
Before bonding occurs, the atoms have thirteen hybridized orbitals. After bonding, there are six hybrid orbitals in HNO₃.
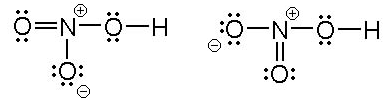
The Lewis structure of HNO₃ shows that it is a resonance hybrid of two structures. The N atom has steric number SN = 3. The electron geometry is trigonal planar. The N atom is sp² hybridized.
The O bonded to H has SN = 4 (two bonding pairs and two lone pairs). The electron geometry is tetrahedral. This O atom is sp³ hybridized.
Of the two remaining O atoms, one has SN = 3 and one has SN = 4. Since both atoms are equivalent, both must have SN = 3. These O atoms are sp² hybridized.
At the moment of bonding, there are 3 + 4 + (2×3) =13 hybridized orbitals.
After bonding has occurred, some of the atomic orbitals have overlapped to form atomic orbitals. The only remaining hybridized atomic orbitals are those that contain the six lone pairs on the O atoms.


