What hybrid orbitals are used by phosphorus in the PCl4+ cations?
1 Answer
Oct 4, 2014
Phosphorus uses sp³ orbitals in PCl₄⁺.
1. Draw the Lewis structure.

2. Use VSEPR Theory to predict the orbital geometry.
This is an AX₄ ion. It has 4 bonding pairs and no lone pairs. The bonds should point toward the corners of a regular tetrahedron.
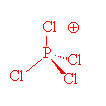
3. Use the orbital geometry to predict the hybridization.
Orbitals that point toward the corners of a regular tetrahedron are sp³ hybridized.


