Equivalent Hydrogens
Key Questions
-
Answer:
When two hydrogen atoms are equivalent, their signals coalesce to give a single peak with twice the area.
Explanation:
If the two hydrogens are non-equivalent but close enough to split each other, you get an AB pattern, a doublet of doublets.
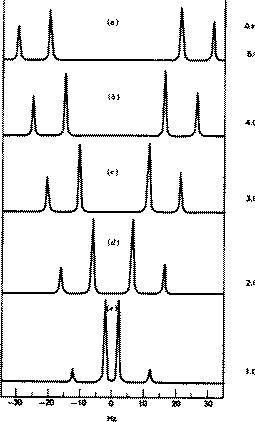
As they become more nearly equivalent, the inner peaks increase in intensity and move closer to each other.
At the same time, the outer peaks decrease in intensity.
At the extreme, when the two hydrogen atoms are equivalent, the inner peaks coalesce to a single peak in the centre, and the intensity of the outer peaks decreases to zero.
-
Answer:
I take it you are asking this on the basis of an NMR experiment?
Explanation:
Protons and carbon nuclei exchanged (only) by a mirror plane, are said to be enantiotopic (i.e. interchanged by an improper axis of rotation). Enantiotopic nuclei are equivalent (i.e. isochronous), and should give rise to the same chemical shifts in all achiral NMR experiments. Should a homochiral NMR solvent be used (i.e. an optically active NMR solvent, which do exist), their interaction with the enantiotopic nuclei is diastereotopic, and the enantiotopic nuclei may be differentiated and become non-equivalent.
-
How can I calculate equivalent hydrogens?
One way is to replace each H with a different group and see if you get a different compound.
Example 1. Identify the equivalent hydrogens in 2-methylpropane.

If I replace any of the nine CH₃ atoms with a Cl, I'll always get the same compound: 1-chloro-2-methylpropane. So the nine CH₃ atoms form one set of nine equivalent hydrogen atoms.
If I replace the H atom on C-2, I'll always get a different compound: 2-chloro-2-methylpropane. So this lone H atom forms a second set consisting of 1 hydrogen atom by itself.
**Example 2. ** Identify the equivalent hydrogens in methyl acetate.
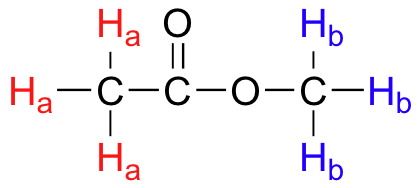
If I replace any of the red CH₃ atoms, I'll always get the same compound: methyl chloroacetate. So the red
#"H"_"a"# atoms form one set of three equivalent hydrogen atoms.If I replace any of the blue CH₃ atoms, I'll always get a different compound: chloromethyl acetate. So the blue
#"H"_"b"# atoms form a second set of three equivalent hydrogen atoms.Now that you have the idea, try to identify the equivalent H atoms in ethyl acetate, CH₃COOCH₂CH₃, and the isomeric chloropropanes, C₃H₅Cl.