Why do we need to identify proton equivalence in the first place?
1 Answer
Jan 9, 2015
We need to identify proton equivalence because it helps to predict the number of signals in an NMR spectrum.
Suppose you had to identify a compound with molecular formula C₃H₇Br from the following NMR spectra.
Spectrum 1.
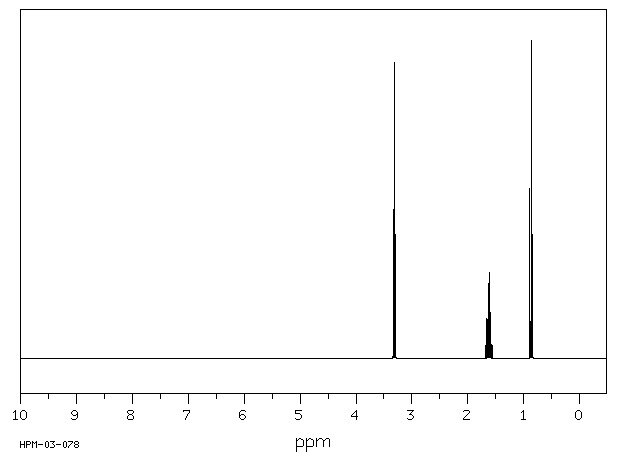
Spectrum 2.

Spectrum 1 has three signals, while spectrum 2 has two signals.
The possible structures are 1-chloropropane, CH₃CH₂CH₂Cl, and2-chloropropane, CH₃CHClCH₃.
You know that 1-chloropropane has three sets of equivalent hydrogens.
The two CH₃ groups in 2-chloropropane are equivalent. This molecule has only two sets of equivalent hydrogens.
Which isomer corresponds to which spectrum?
(Hint: Spectrum 1 is the 1-chloropropane.)

