Aromatics
Key Questions
-
Aromaticity is important because it makes molecules more stable.
Aromatic compounds play important roles in biochemistry and in industry.
IMPORTANT BIOCHEMICAL AROMATICS
Aromatic compounds play key roles in the biochemistry of all living things
Amino Acids
Amino acids are the building blocks of proteins. Four of them — histidine, phenylalanine, tyrosine, and tryptophan — are aromatic.

Nucleotides
Five nucleotides make up the sequence of the genetic code in DNA and RNA. All five — adenine, guanine, cytosine, thymine, and uracil — have aromatic rings. They contribute to the stability of DNA and RNA.
Heme
The molecule heme contains a ring system with 22 π electrons. According to Hückel's 4
#n# + 2 rule, it is aromatic (#n# = 5).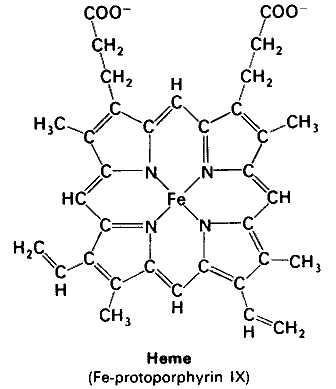
IMPORTANT INDUSTRIAL AROMATICS
Important commercial aromatic compounds include benzene, toluene, 1,2-, and 1,4-dimethylbenzene.
These in turn are the starting materials for compounds such as styrene, phenol, aniline, polyesters, and nylon.
-
Benzene is an aromatic , ringed hydrocarbon (since it contains only C & H )
Molecular Formula - C6H6 ( 6 C's arranged in ring with one H each )
Benzene acts as highly reacting & attracting species for electrophiles ( electron - loving species ) due to delocalization of pie electrons . This phenomenon is termed as Resonance ! Benzene is so famous due to resonance acting in it .
As per structure :
The C–C bond lengths are greater than a double bond, (135 pm), but shorter than a single bond, (147 pm).
This intermediate distance is consistent with electron delocalization: (the electrons for C–C bonding are distributed equally between each of the six carbon atoms.)The molecule is planar. Each carbon is sp2 hybridised !