Why is benzene an aromatic hydrocarbon?
1 Answer
Benzene is an aromatic hydrocarbon because it obeys Hückel's rule.
Originally, benzene was considered aromatic because of its smell: it has an "aromatic" odor.
It is now considered aromatic because it obeys Hückel's rule:
In the case of benzene, we have 3 π bonds (6 electrons), so
Another example is naphthalene, with two fused benzene rings.
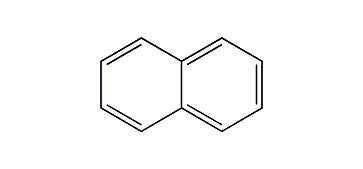
It has 5 π bonds and 10 electrons.
So, naphthalene is aromatic…
This link should help you.
http://www.wikiwand.com/en/H%C3%BCckel%27s_rule
Best regards;
Hossam…


