Calculating Energy in Electrochemical Processes
Key Questions
-
The Nernst equation is an equation that relates the reduction potential of a half-cell at any point in time to the standard electrode potential, temperature, activity, and reaction quotient of the underlying reactions and species used.
It is named after the German physical chemist who first formulated it, Walther Nernst.
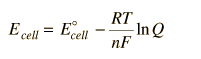
-
The Nernst equation
#E^0=E^theta+(RT)/(nF)ln("Oxidants"/"Reductant")# It has other forms; but basically, it shows us the relationship between Electrode potential of a half cell and Temperature.
It is a direct relationship. That is, increasing the temperature if half cell, leads to a rise in electrode potential.