Single Replacement Reactions
Key Questions
-
In a single-displacement reaction, one element displaces another element in a compound.
The general equation for a single-displacement reaction is:
A + B-C → A-C + BA and B must be either different metals (including H) or different halogens.
Chemists have devised an Activity Series. It lists the metals and nonmetals in decreasing order of chemical activity. An element that is higher in the series will displace an element that is below it. This enables chemists to predict which combinations will undergo single displacement reactions.
For example, Mg is above H in the series. We predict that Mg should replace the H in HCl, forming MgCl₂ and gaseous H₂.
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
Also, Cu is above Ag. We predict that Cu should replace the Ag in AgNO₃, forming Cu(NO₃)₂ and solid Ag.
Cu(s) + 2AgNO₃(aq) → Cu(NO₃)₂(aq) + 2Ag(s)
Cl is above I. We predict that Cl₂ should replace the I in KI, forming KCl and I₂.
Cl₂(aq) + 2KI(aq) → 2KCl + I₂(aq)
Will there be a reaction between Br₂ and KCl? Br is below Cl in the series, so we predict no reaction.
Video comparing the activity of three metals (Cu, Mg and Zn)
-
A decomposition reaction is one where a compound is broken down into its constituent chemical species:
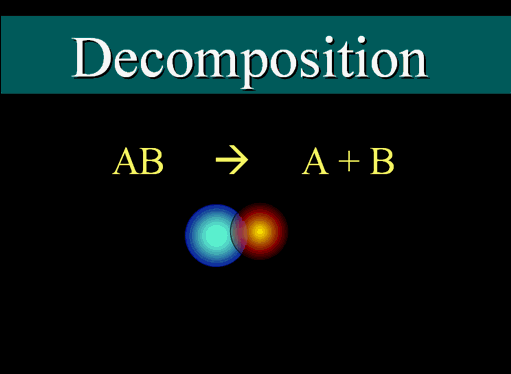
For example:#2NaCl -> 2Na^+ + Cl_2^-# The
#NaCl# was broken down into the constituents#Na^+# and#Cl_2^-# --(side note: the
#Cl# is diatomic that explains the#2# )There are two types of replacement reactions, observe the differences:
Single Replacement:#AB + C -> AC + B# Double Replacement:
#AB + CD -> AD + CB# -
Answer:
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound.
Explanation:
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound.
There are two types of single replacement reactions:
A metal replaces another metal that is in solution:
#"A" + "BC" → "B" + "AC"#
Example:#"Zn" + "CuCl"_2→ "Cu" + "ZnCl"_2# A halogen replaces another halogen that is in solution:
#"A" + "BC" → "C" + "BA"#
Example:#"Br"_2 + "2KI" → "I"_2+ "2KBr"# To determine whether a given single replacement will occur, you must use an “Activity Series” table.
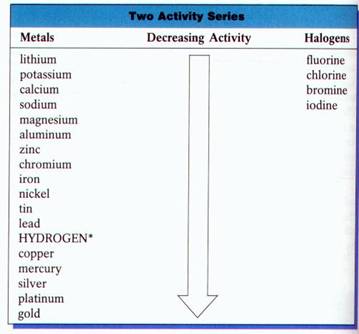
If the metal or the halogen is above the element it will replace based on the activity series, a single displacement reaction will occur.
Examples:
#"Mg" + "2HCl" → "H"_2 + "MgCl"_2# (#"Mg"# is above#"H"# )#"Cl"_2 + "2NaBr" → "Br"_2 + "2NaCl"# (#"Cl"# is above#"Br"# )#"Cu" + "AlCl"_3→ "no reaction"# (#"Cu"# is below#"Al"# )The video below summarizes an experiment conducted to compare the activities of three metals (
#"Mg, Cu"# and#"Zn"# ).