Why is benzene planar and cyclohexane nonplanar?
1 Answer
Short answer: Benzene is planar because its carbon atoms are
Explanation:
Benzene
The structure of benzene is

Each carbon atom is bonded to three other atoms, so it is
The interior angles of a regular hexagon are 120°.
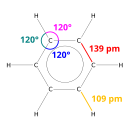
This exactly matches the
Cyclohexane
The structure of cyclohexane is
Each carbon atom is bonded to four other atoms, so it is
If the carbon atoms in cyclohexane were arranged as a planar hexagon, the bond angles would have to be 120°.
This would introduce a large amount of angle strain (and other types of strain) into the molecule.
The molecule can relieve this strain if it puckers into a three-dimensional chair shape.

This brings the bond angles back to 109.5 ° and minimizes all the strains in the molecule.


