Why did Baeyer predict that cyclopentane would be the most stable of the cycloalkanes? Was that correct?
1 Answer
Baeyer predicted that cyclopentane would be the most stable of the cycloalkanes because its bond angles of 108 ° are closest to the tetrahedral angle of 109.5 °. He was wrong.

He also predicted that cyclohexane, with bond angles of 120 °, would be less stable than cyclopentane.
And he predicted that the stability of larger rings would decrease as the number of sides increased.
But cyclohexane is more stable than cyclopentane. And the stability of compounds does not decrease as the number of sides increases.
Baeyer incorrectly assumed that all molecules are planar. He didn't recognize that they could pucker to avoid the torsional strain caused by eclipsed hydrogens.
Cyclopentane can relieve most of the torsional strain by puckering, but this increases ring strain.
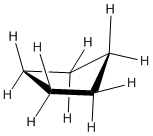
Cyclohexane can pucker into a chair form to relieve both torsional and ring strain.

But larger rings such as cyclooctane can also pucker to relieve torsional and ring strain.


