Which one of the following is expected to exhibit resonance? (A) #CO_2# (B) #NH_4^(+)# (C) #HCN# (D) #NO_2""^(-)#
1 Answer
The expected answer is probably D)
Explanation:
Both
To determine if resonance is possible, you draw the Lewis structure and then see if you can put the electrons in different locations.
A)
The Lewis structure for

There are two other resonance contributors, but they involve charge separation, so they are minor contributors.
B)
The Lewis structure of
This is the only Lewis structure — no resonance.
C)
The Lewis structure of

You can draw a structure with a
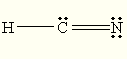
D)

