How do resonance structures and isomers differ?
1 Answer
Short answer: Resonance structures differ in the location of electrons. Isomers differ in the location of atoms.
Explanation:
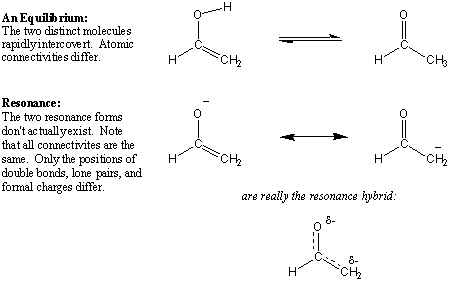
Resonance structures represent the same compound.
For example, acetone has two resonance contributors.
They differ only in that a pair of π electrons has moved onto the oxygen atom. Only the electrons move, not the atoms.
The molecule is a resonance hybrid of the two structures.
Dimethyl ether and ethanol are isomers. They have different chemical and physical properties.
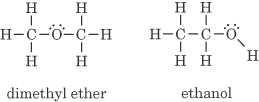
Converting dimethyl ether to ethanol requires breaking a C-O and a C-H σ bond and forming new C-C and O-H σ bonds. The atoms change positions and form two different compounds.