What is a delocalized pi bond?
1 Answer
Nov 20, 2016
A delocalized π bond is a π bond in which the electrons are free to move over more than two nuclei.
Explanation:
In a molecule like ethylene, the electrons in the π bond are constrained to the region between the two carbon atoms.

We say that the π electrons are localized.
Even in penta-1,4-diene, the π electrons are still localized.
The
(From iverson.cm.utexas.edu)
However, in buta-1,3-diene, the two orbitals can overlap, and the π electrons are free to spread over all four carbon atoms.
We say that these π electrons are delocalized.
In benzene, the π electrons are delocalized over all six atoms of the ring.
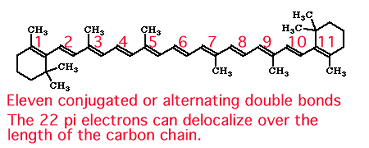
In β-carotene, the π electrons are delocalized over 22 carbon atoms!