How many molecular orbitals are in #O_2#?
1 Answer
Jan 27, 2015
O₂ has an infinite number of molecular orbitals, but only nine of them are occupied in the ground state.
The molecular orbital diagram for O₂ is
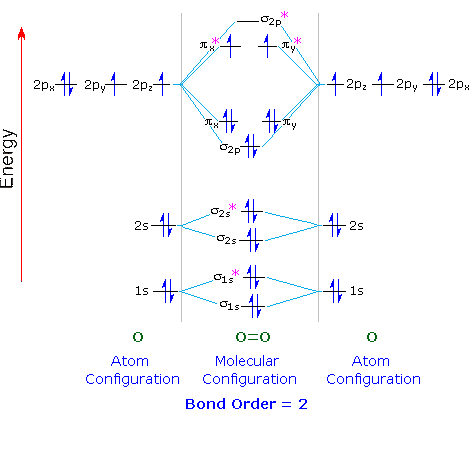
Each O atom contributes 8 electrons to the O₂ molecule.
You use the Pauli Exclusion Principle and Hund's Rule to add the 16 electrons to the molecular orbitals in an Aufbau process.
The molecule then has 5 fully occupied bonding molecular orbitals, 2 fully occupied antibonding orbitals, and 2 half-filled molecular orbitals.
The video below describes how to construct the molecular orbital diagram for O₂. Note that it covers only the orbitals that contain the valence electrons.

