What determines carbocation stability?
1 Answer
The three factors that determine carbocation stability are adjacent (1) multiple bonds; (2) lone pairs; and (3) carbon atoms.
(1) Adjacent multiple bonds
An adjacent π bond allows the positive charge to be delocalized by resonance.
Thus,
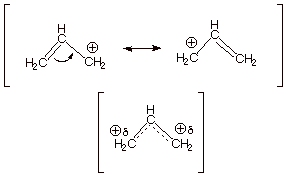
Resonance delocalization of the charge through a larger π cloud makes the cation more stable.
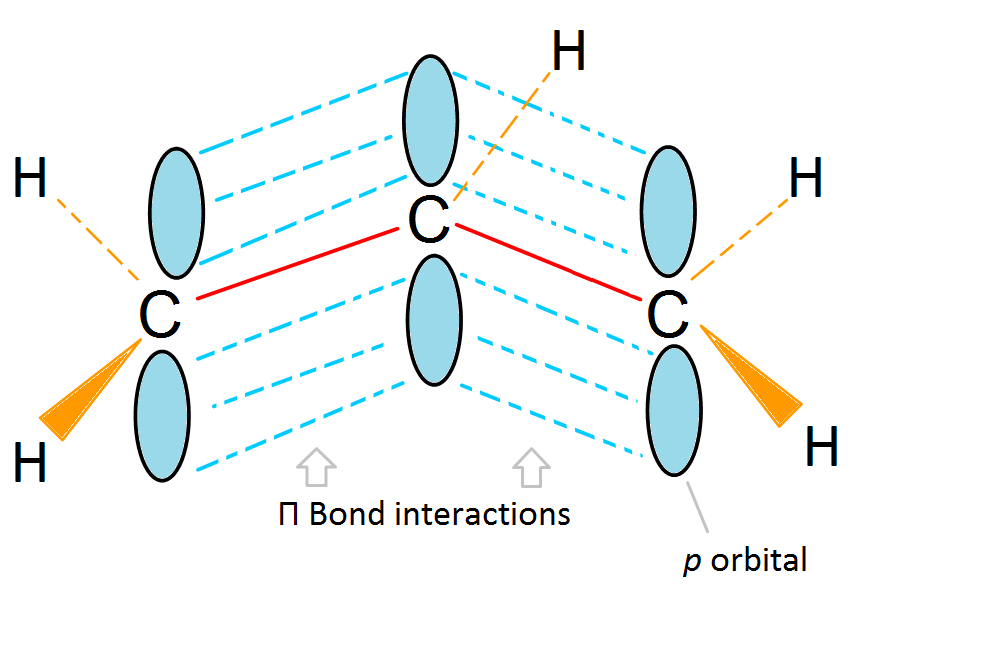
(2) Adjacent lone pairs
A lone pair on an adjacent atom stabilizes a carbocation.
Thus,
The cation is more stable because the charge is spread over two atoms.
(3) Adjacent carbon atoms
The stability of carbocations increases as we add more carbon atoms to the cationic carbon.
For example,
The stabilization is explained by a type of resonance called hyperconjugation.
The

The delocalization of charge stabilizes the carbocation.
Thus, the more alkyl groups there are surrounding the cationic carbon, the more stable it becomes.
That gives us the order of stability:


