What are some examples of trigonal #sp^2# hybrids?
1 Answer
Examples of
Explanation:
In order for an atom to be
Three regions of electron density
In order for a molecule to have a trigonal planar molecular geometry, its central atom must be bonded to three atoms and have no lone pairs of electrons present.
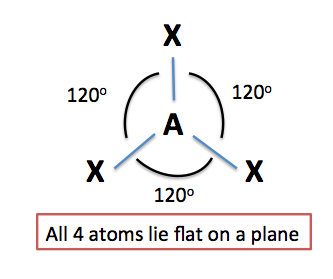
In other words, all the three regions of electron density that surround the atom must be bonds to other atoms.
Boron trifluoride,
In both cases, the central boron atom is bonded to three other atoms and has no lone pairs present.
 )
)

Other examples are formaldehyde,
 )
)
and the carbonate ion,
 )
)

