How do I rank the following compounds from lowest to highest boiling point: calcium carbonate, methane, methanol (CH₄O), dimethyl ether (CH₃OCH₃)?
1 Answer
The order of boiling points is:
Explanation:
The order of strengths of intermolecular forces is:
Compounds with stronger intermolecular forces have higher boiling points.
The strongest intermolecular force in each of the compounds is:
Next comes methanol,
Methanol has strong hydrogen bonds. It will have the next highest boiling point.
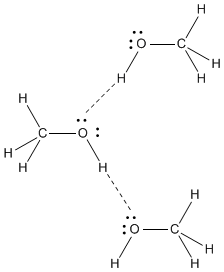
Dimethyl ether,
The
Dipole-dipole forces are not as strong as hydrogen bonds, so dimethyl ether has a lower boiling point than methanol does.
Finally, the
It has only weak London dispersion forces,

The order of boiling points is:
Here's a good video on ordering compounds according to their intermolecular forces and boiling points.

