How would you explain the electrophilic aromatic substitution with a diagram of a reaction mechanism?
1 Answer
Electrophilic aromatic substitution requires of an aromatic reactant, a pre-electrophile and an electrophile maker.
Explanation:
The reaction begins with a 'pre-step' which involves the electrophile maker turning the pre-electrophile into a very reactive electrophile.
Only reactive electrophiles can cause a reaction because aromatic rings are very stable.
The aromatic rings acts as a nucleophile.
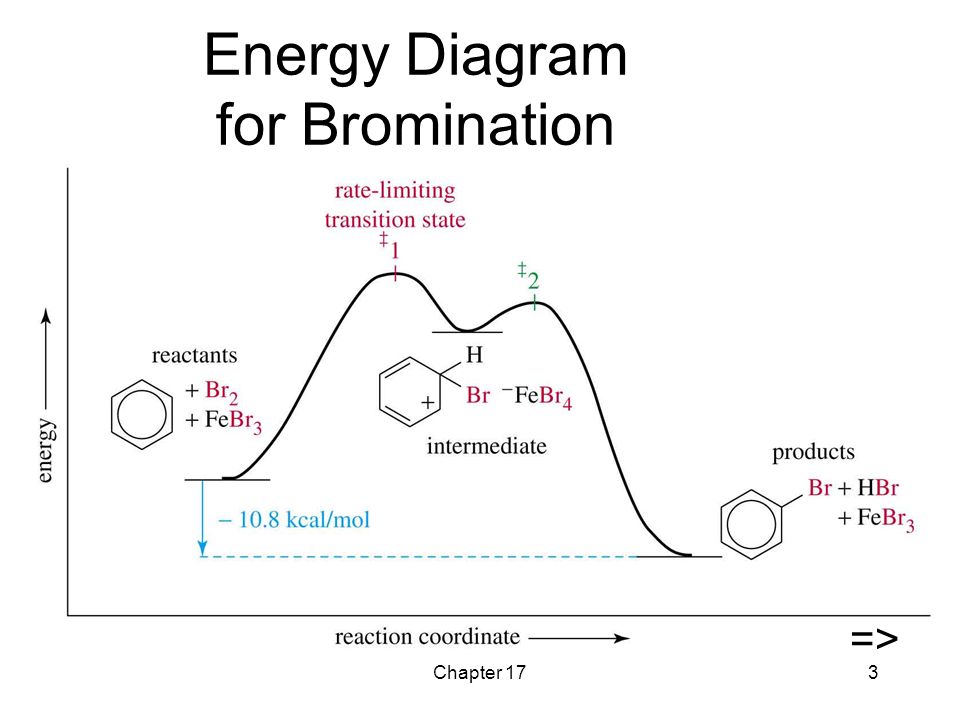
I will take the bromination of benzene as an example:

1. The reaction starts with the production of a good electrophile by a catalyst.
For the bromination of benzene reaction, the electrophile is the

2. The electrophile attacks the π electron system of the benzene ring to form a non-aromatic carbocation intermediate.

3. The positive charge on the carbocation is delocalized throughout the molecule.

4. The aromaticity is restored by the loss of a proton from the atom to which the bromine atom (the electrophile) has bonded.

5. Finally, the proton reacts with the

Here's an energy diagram that corresponds for the bromination of benzene.