How to locate the position of element with atomic number 120 which is not yet discovered ?
1 Answer
You use the order of filling orbitals and your knowledge of the Periodic Table to locate Element 120.
Explanation:
The known elements
The last known element in the Periodic Table is Element 118,
(From Fotolia,com)
It is in the lower right-hand corner of the Periodic Table, at the end of Period 7 and the bottom of Group 18.
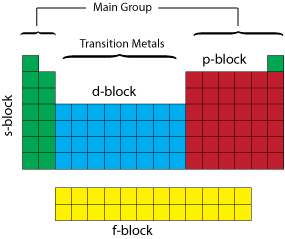
It is the last element in the
Where is element 120?
We use the order of filing atomic orbitals.
(Adapted from Wikibooks)
It shows that the next electrons will go into the
The
That’s just enough electrons to get us from
(From www.chemicool.com)
You will find it under radium (at. no. 88), so it should be an alkaline earth metal.

