How many resonance structures are there for carbon trioxide?
1 Answer
Sep 22, 2014
There are three different isomers of CO₃, so we must consider each isomer separately.
Carbon trioxide (CO₃) is an unstable oxide of carbon. It is quite different from the stable carbonate ion, CO₃²⁻.
The three isomers of carbon trioxide are
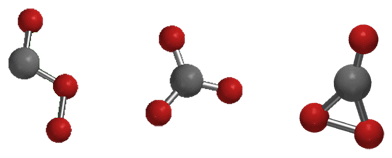
Structure 1 has two unpaired electrons and only one resonance contributor.
Structure 2 has two unpaired electrons and three resonance contributors.
Structure 3 has no unpaired electrons and one contributor. All three isomers are unstable, but this one is the most stable of the three.

