How many bonding orbitals are there in #"AsH"_3#?
1 Answer
Aug 15, 2014
There are three bonding orbitals in AsH₃.
If you draw the Lewis structure and the VSEPR shape, you find that AsH₃ has a trigonal pyramidal shape.
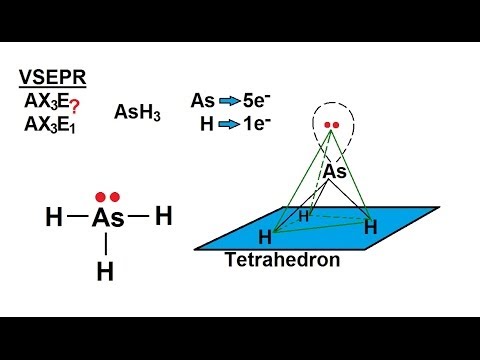
The molecule is sp³ hybridized.
There is a lone pair of electrons in one of the sp³ orbitals.
The three As-H σ bonds form by overlapping of the sp³ orbitals with the 1s orbitals of the H atoms.
According to molecular orbital theory, each time you form a bonding σ orbital you also form an antibonding σ* orbital.
So AsH₃ has seven molecular orbitals:
- 3 bonding As-H σ orbitals
- the nonbonding sp³ orbital containing the lone pair
- three antibonding σ* As-H orbitals
The three bonding orbitals and the one nonbonding orbital contain the eight valence electrons in AsH₃.

