How do I fill bonding and antibonding orbitals?
1 Answer
Once you have drawn and labelled molecular orbitals, you fill them in the same order as you fill atomic orbitals.
The rules you use for filling atomic orbitals are:
1. Aufbau Principle
You place electrons in the lowest energy orbitals available.
2. Pauli Exclusion Principle
No orbital may hold more than two electrons, and they must have opposite spin.
3. Hund's Rule
Every orbital in a subshell must contain only one electron before any orbital can have two electrons. All electrons in singly occupied orbitals must have the same spin.
You use the same rules to fill molecular orbitals.
Let's apply this to the molecular orbital diagram of oxygen.
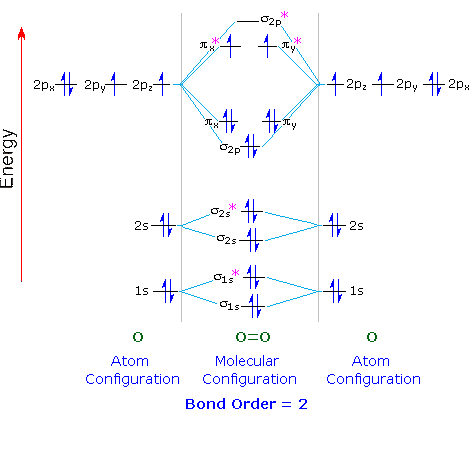
Each O atom contains 8 electrons, so we have 16 electrons to put into the molecular orbitals of O₂.
We put two electrons into each of the σ1s, σ1s, σ2s, σ2s, and σ2p (10 electrons).
The next four electrons follow Hund's Rule and go in order into the
The remaining two electrons follow Hund's Rule and go in order into the
An oxygen molecule has two unpaired electrons.

