How do you know the hybrid orbitals of a compound? For example, what are the hybrid orbitals of #CH_2Cl_2#, #C_2H_4#, and #C_2H_2#?
1 Answer
The hybrid orbitals in
Explanation:
In each case, you must draw the Lewis structure, determine the VSEPR geometry, and then assign the corresponding hybridization.
The Lewis structure of
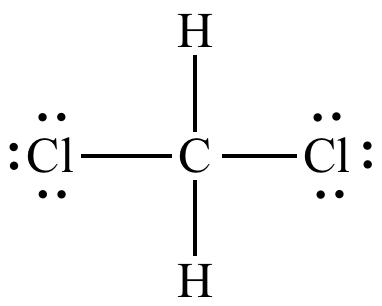
There are four electron domains around the carbon atom, do the electron geometry is tetrahedral.

The hybridization that corresponds to a tetrahedral geometry is

The Lewis structure of
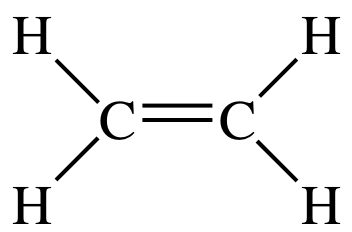
There are three electron domains around each carbon atom, so the electron geometry is trigonal planar.
The hybridization that corresponds to a trigonal planar geometry is

The Lewis structure of

There are two electron domains around each carbon atom, so the electron geometry is linear.

The hybridization that corresponds to a trigonal planar geometry is
(From Learnnext)

