How do you identify aromatic rings?
1 Answer
An aromatic ring must be a planar, cyclic, system containing (4n + 2) π electrons in alternating single and double bonds with no intervening saturated carbon atoms.
Explanation:
Hückel's rule states that an aromatic system must have (
Examples
Thus, benzene is aromatic because it satisfies Hückel's rule.
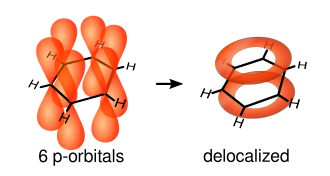
The cyclopropenyl cation is also aromatic.
A ring with 4 pi electrons is not aromatic

n is a fraction.
Thus, cyclobutadiene doesn't follow Hückel's rule and is not aromatic.


