How do you draw the sp3 orbital?
1 Answer
Dec 19, 2016
For convenience, most chemists usually draw it as an elongated teardrop.
Explanation:
It looks like this:
(Adapted from OChemPal)
The pointed end is attached to the nucleus and points away from it.
In fact, the same shape usually serves for
There are four equivalent
The actual shape of each orbital is more like that of a stubby mushroom, as shown below.
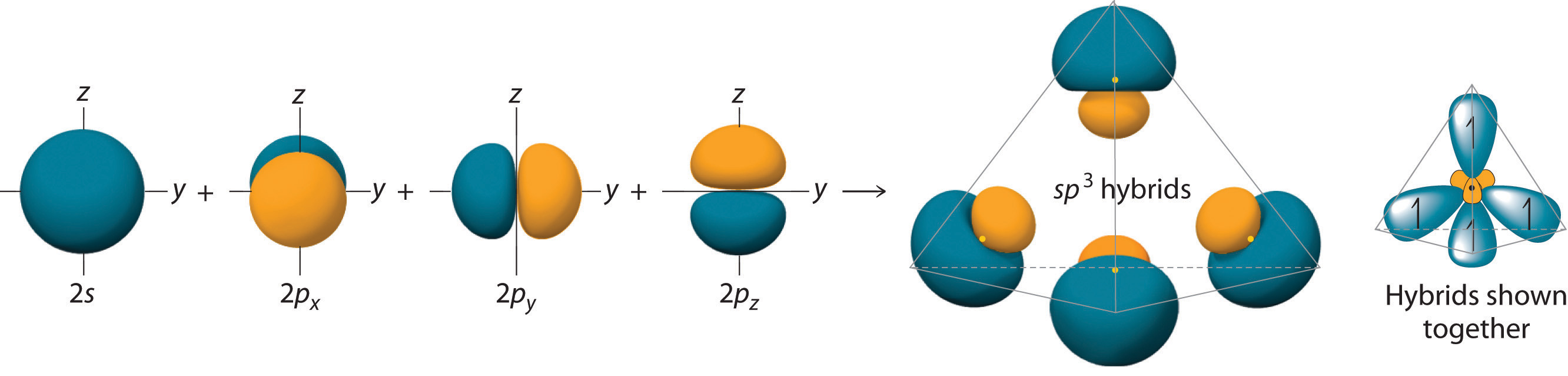
Each lobe has a large lobe and a small one, and the big lobe is much fatter than a teardrop.
Thus, it would be more correct to draw the orbital like this:

Even so, most chemists omit the small lobe and use a single teardrop shape unless they wish to draw attention to the small lobe.

