How can I draw antibonding orbitals?
1 Answer
Jul 20, 2014
You add a node perpendicular to the internuclear axis and draw most of the electron density pointing away from the two nuclei.
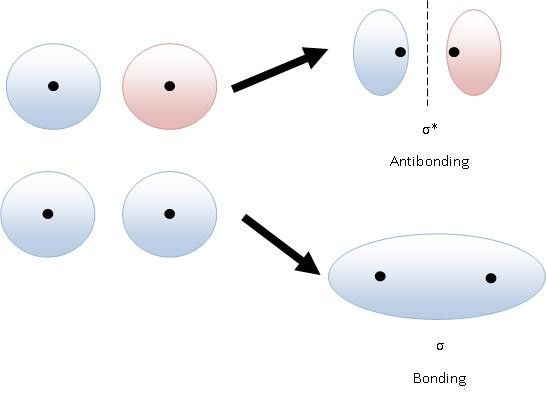
A sigma orbital has no nodes. Most of the electron is between the two nuclei. An antibonding sigma orbital has a node. There is a little electron density between the nuclei, but most of it points in the opposite direction.
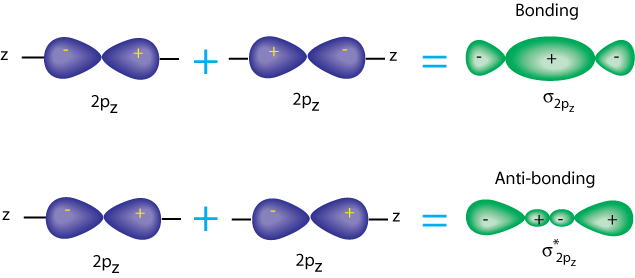
It is the same with p-p sigma orbitals. The p orbitals each have a nodal plane, but now the antibonding orbital has another node. Note that there is still a little electron density between the nuclei, but most of it is outside.

The same principles apply to p-p pi orbitals.

