How can I draw the molecular formula for an unsaturated alkyl chloride #C_5H_9Cl# with optical activity and E, Z – isomerism?
1 Answer
First, draw the possible (
Explanation:
Step 1. Draw the isomeric pentenes.
There is only one pentene that can have (
You can have (
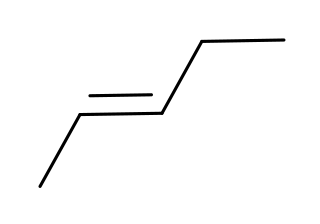
or (
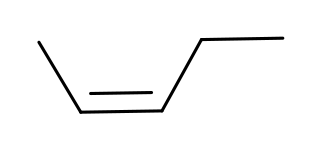
Step 2. Identify the prochiral carbon atoms.
A
It must be bonded to exactly three different groups before any change is made.
In pent-2-ene, C-4 is bonded to a methyl group, a propenyl group, and two hydrogens.
This fits the criterion of being bonded to exactly three different groups, even though one group is represented twice.
If we replace one
So there are four possibilities:
(
and (

