How can I draw 3–methylpent–2–ene, (#CH_3CH = C(CH_3) CH_2CH_3#), and know if it shows E-Z isomerism?
1 Answer
Aug 24, 2015
First you expand the condensed structural formula to a bond-line view, and then you check whether there are two different groups on each end of the
Explanation:
Step 1. Draw the bond-line structure.
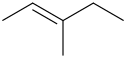
Step 2. Check for two different substituents on each end.
In the structure above, the left hand
The right hand end has a
The compound shows
In the above structure, the
Since the two high-priority groups are on opposite sides of the double bond, the compound is (

