How can I draw an elementary reaction in a potential energy diagram?
1 Answer
You join the potential energy diagrams into one diagram.
Explanation:
A potential energy diagram represents the energy pathway for an elementary reaction — a single-step process.
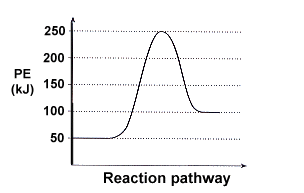
Many reactions take place in a series of steps.
For example, the starting materials (
(
We would write the reaction as occurring in two steps:
Step 1:
Step 2:
Overall:
Step 1 is an elementary reaction, so we draw a potential energy diagram going from
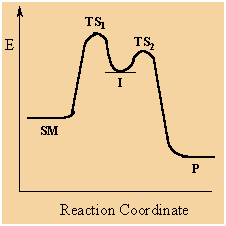
Step 2 is another elementary reaction, so we draw a potential energy diagram going from
But the starting point for Step 2 is the ending point for step 1, so we join the two diagrams together to make a double-hump curve as shown above.
Each hump represents the transition state for a separate elementary reaction.

