How can I draw a simple energy profile for an exothermic reaction in which 100 kJ mol-1 is evolved, and which has an activation energy of 50 kJmol-1?
1 Answer
You can start with a generic potential energy diagram for an exothermic reaction.
 )
)
A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed.
So, the activation energy is the minimum amount of energy required for a reaction to take place. In your case, you need at least
The heat evolved during the reaction,
Here's what an energy diagram for such a reaction could look like
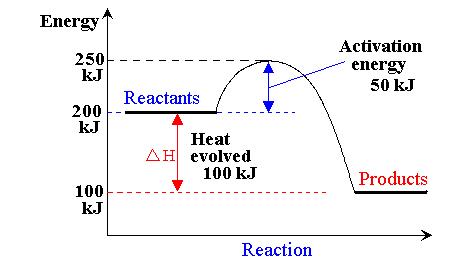
I've assumed the energy level of the reactants to be
In an exothermic reaction, the products will always be lower in energy than the reactants; in this case, the products are at

