How can I convert a bond line notation for ethane molecule to Newman projection?
1 Answer
You convert the bond-line structure to a wedge-dash structure. Then you convert the wedge-dash structure to a Newman projection.
Explanation:
The bond line structure of ethane is
The wedge-dash structures for the staggered and eclipsed conformations are:
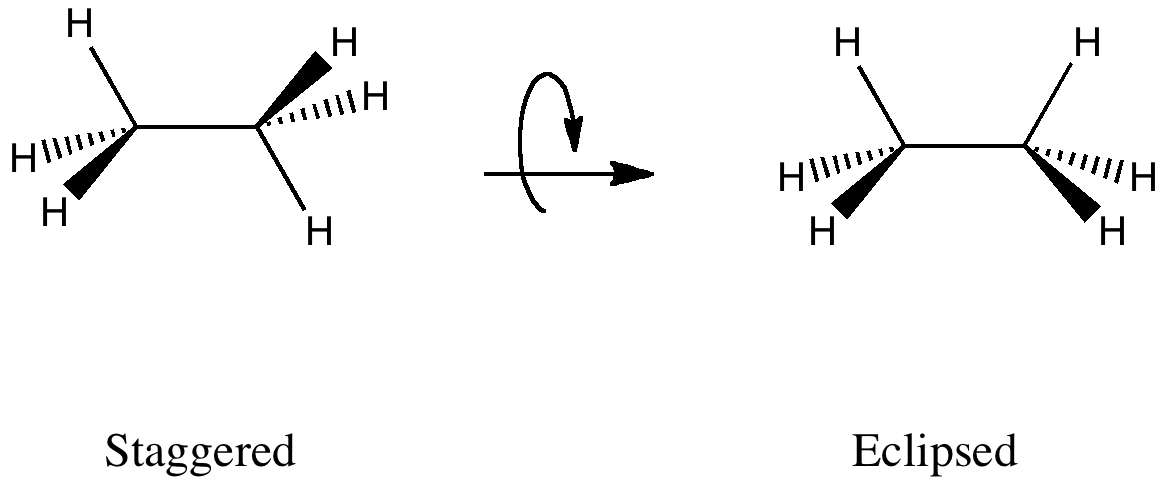
Here's how to convert the wedge-dash structures to Newman Projections.
Staggered Ethane
Step 1. View the molecule along the C1-C2 axis with your eye at the left-hand end.
The closest bonds make an inverted Y, so you will choose that template for your projection.
Step 2. Place the groups onto your template.
All groups on the carbon atoms are H atoms.

And you have your staggered Newman projection for ethane.
Eclipsed Ethane
Step 1. Draw two eclipsed Newman templates.
Step 2. View the molecule along the C1-C2 axis with your eye at the left-hand end.
The closest bonds make an inverted Y, so you will choose the second template for your projection.
Step 3. Place the groups onto your template.
All groups on the carbon atoms are H atoms.
And you have your eclipsed Newman projection for ethane.

