When two atomic orbitals overlap, they form two molecular orbitals, of which one is bonding and one is antibonding.
For example, two #p# orbitals overlap to form a #π# (bonding) and a #π#* (antibonding) orbital.
We can see this in ethene, buta-1,3-diene, and benzene.
Ethene
![Ethene]()
(from www.dlt.ncssm.edu)
Each time you go up an energy level, you get another node.
Buta-1,3-diene
If you use four atomic #p# orbitals to make the molecular orbitals in butadiene, you get four new #π# orbitals.
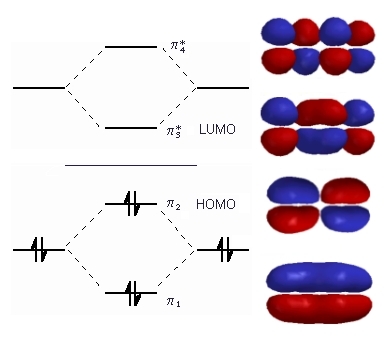
Each level up has one more node.
The four #p# electrons go into the two bonding orbitals, one of which has a node.
Benzene
The six #p# orbitals in benzene combine to form six #π# orbitals, of which three are bonding and three are antibonding.
![Benzene]()
(from jwhitesell.ucsd.edu)
The lowest-energy orbital, #π_1#, has zero nodes and has the familiar doughnut shape. It holds two #π# electrons.
#π_2# and #π_3# are degenerate bonding orbitals with one node. The node in #π_2# is at right angles to the node in #π_3#. Each orbital contains two electrons.
#π_4# and #π_5# are degenerate antibonding orbitals with two nodes at right angles to each other.
#π_6# is an antibonding orbital with three nodes.


