Are the contributing structures of resonance structures isomers?
1 Answer
The contributors to resonance structures are not isomers.
Isomers are molecules with the same molecular formula but different chemical structures. For example, propan-1-ol, propan-2-ol, and methoxyethane are isomers

The structures of isomers differ in the positions of their atoms. Isomers exist as separate molecules, with different physical and chemical properties.
The structures that contribute to a resonance hybrid do not exist. They are imaginary structures that we invent to explain the properties of compounds.
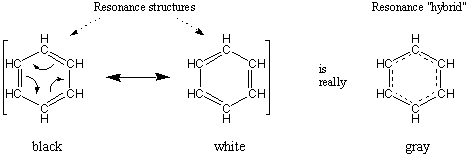
For example, we say that benzene is a resonance hybrid of two contributing structures. Let's call them "black" and "white".
We cannot observe these "black" and "white" contributors. The molecule does not oscillate back and forth between these structures. Benzene is not "black" half the time and "white" the other half.
Benzene is always "grey". It does not have alternating single and double bonds of different lengths. Instead, the C=C bonds are all identical, with bond orders equal to 1.5.
Contributing structures differ only in the position of electrons.
Only the electrons move. The atoms stay in the same place.
Thus, contributing structures are not isomers.
Hope this helps.

