Question #cabb8
1 Answer
Because of orbital hybridization.
Explanation:
Phosphorus,
Now, an isolated phosphorus atom in its ground state can only use
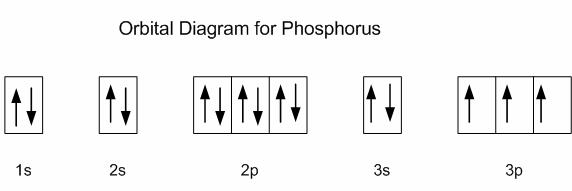
This is why phosphorus is said to have a valency of
However, phosphorus can promote an electron from the occupied
These orbitals will hybridize to form
- the 3s orbital
- the three 3p-orbitals
- one 3d-orbital
 )
)
The atom can now form five covalent bonds with five chlorine atoms to form phosphorus pentachloride,
 )
)
Phosphorus is able to promote an electron to one of its
In other words, promoting this electron to a

