Cyclohexane Chair Flip
Key Questions
-
Answer:
Chair is more stable than boat because... see explanation
Explanation:
In both conformations chair and boat, the bond angle is
#109.5^@# which eliminates the angular strain in the cyclohexane structure. However, why chair is more stable than boat?The answer is because of the torsional and steric strains which are more present in a boat conformation.
This video explains in details the conformation energy of cycloalkanes:
-
Answer:
Yes
Explanation:
All equatorial substituents become axial and vice versa.
-
Answer:
Just to retire this question.....this is really something you should address with a model....
Explanation:
A cyclohexane ring lies ROUGHLY in the plane, and each CARBON bears an AXIAL substituent, and an EQUATORIAL substituent.
 )
) There are SIX blue equatorial substituents, and SIX red axial substituents....and there is a fairly soft energy of exchange which exchanges the axial and equatorial substituents... If the substituent is LARGER than hydrogen, the conformation with the bulky substituent in the equatorial position is the one that is most energetically stable in that it minimizes the interaction between the axial substituents. And again I stress that you should verify what I say and mean with a model in your hands....
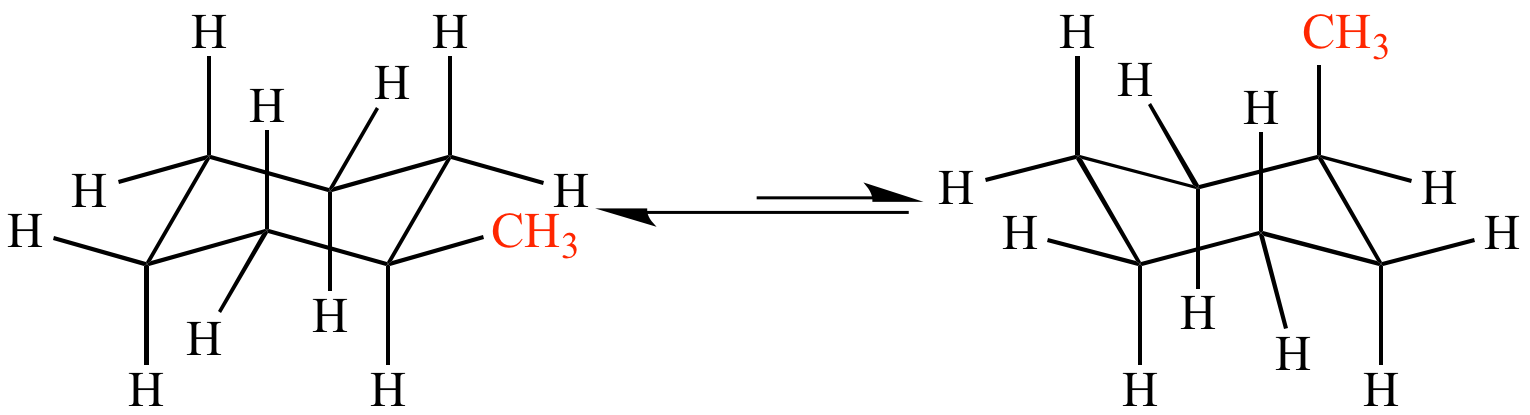
Now when these conformations undergo transition, the axial and equatorial substituents EXCHANGE DIRECTLY.... it is a non-trivial proposition to draw a diagram of the process, and it will repay some practice should you be asked to represent the exchange in an exam...